Measuring the impact of innovation in pediatrics: A framework for improving trial efficiency

Pediatric trials face long-standing challenges across recruitment, adherence, and retention—issues that directly impact timelines, data quality, and cost. Little Journey’s platform was created to address these barriers. As the scope and complexity of digital health innovations grow, so does the need to clearly articulate how and why each component delivers impact.
This white paper, authored by experienced researcher Dr Alex Christensen, sets out a rigorous, evidence-informed framework that maps the pathways between digital features, behavioural mechanisms, and trial outcomes. It explains how Little Journey translates psychological theory, user insight and modular design into measurable improvements for families, sites, and sponsors.
Key insights...
1. The core challenges across pediatric trials
Including the systemic barriers affecting engagement, adherence, and retention, and why these challenges compound uniquely in pediatric studies.
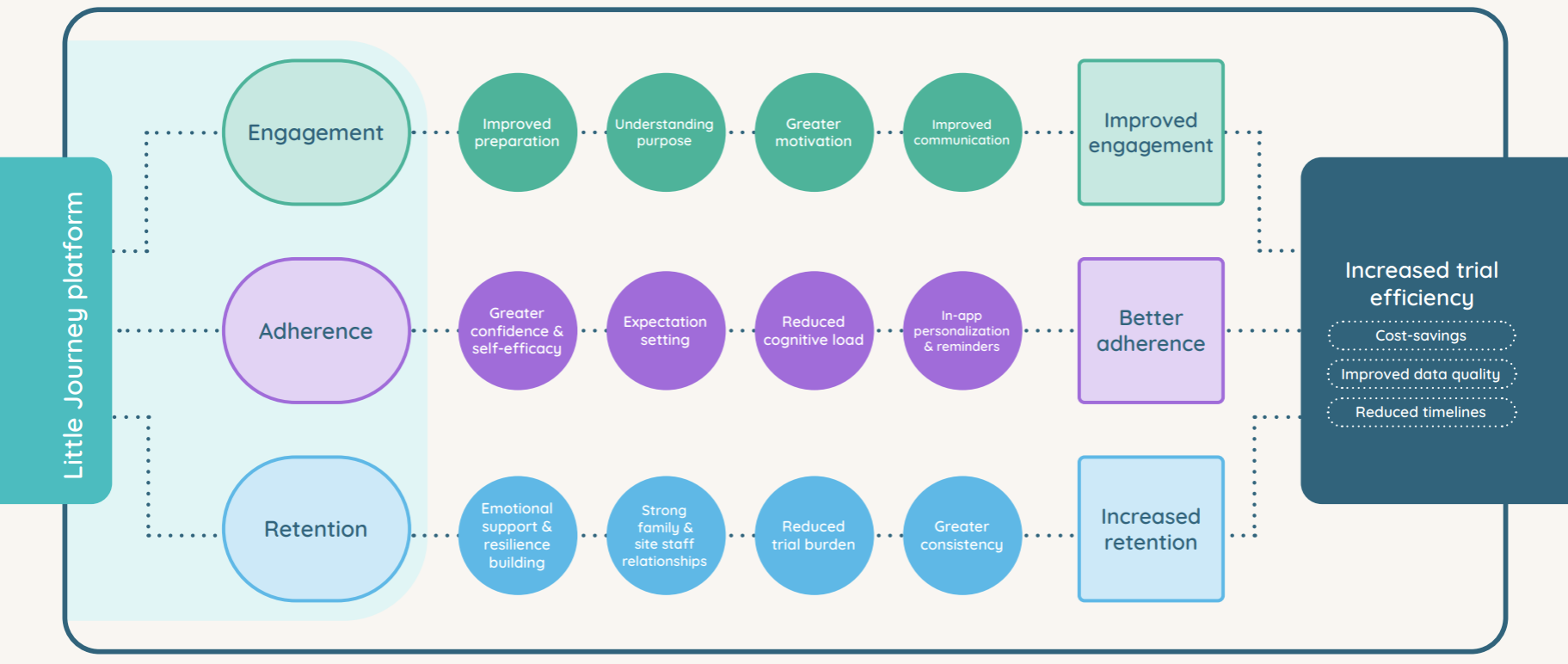
2. How Little Journey’s platform maps to trial outcomes
A clear, visualised pathway showing how platform features drive changes in preparation, confidence, communication, motivation, emotional support, and trust to ultimately improve trial efficiency.
3. The assumptions required for digital interventions to succeed
From digital access and usability, to caregiver trust and site integration. This white paper provides transparency around the foundations necessary for impact.
4. A measurable evaluation framework
Benchmarkable indicators across visit compliance, procedural completion, data quality, experience ratings, and retention.
Why this matters
This framework ensures every element of a digital intervention platform contributes meaningfully to engagement, adherence, and retention - three critical drivers of trial success.
A Theory of Change provides:
-
Clarity on how digital features create behavior change.
-
Confidence for sponsors and regulators through measurable pathways.
-
Consistency across studies while retaining flexibility in configuration.
-
Scalability across therapy areas, age groups, and trial designs.

Download our white paper
Explore how a structured, evidence-based framework can improve engagement, adherence, and retention across your pediatric trials.
About the author
.png?width=1200&height=1200&name=Alex%20Christensen%20Headshot%20(1).png)
Dr Alex Christensen
Alex is an experienced researcher and evaluator, with a PhD in public health and 10 years of experience across academia, the NHS, and industry. Her cross-sector background allows her to speak to both the “why” and the “how” of patient-centred impact. In her role as Evidence Manager, Alex brings methodological rigor and behavioural science expertise make evidence meaningful, inclusive, and commercially relevant for organisations.
