Clinical trials
The first family retention platform for pediatric research
Our highly configurable platform is built on behavioral science and the real-world needs of children, teenagers, and caregivers. We support families from first visit to final data point - helping you deliver studies on time and on budget, ready to bring life-changing treatments to market.
.webp?width=1081&height=1081&name=LJ%20Website%20Update_4.%20Home%20page%20(patients%20%26%20families).webp)
We are proud to support
50,000+
children & families
17+
countries worldwide
9
top global pharma & CROs
Improving trial engagement, adherence and retention at scale
Our scalable platform infrastructure allows us to rapidly deploy a sponsor or study branded app, with content tailored to the therapeutic area, trial protocol, patient population, and local trial site and language.
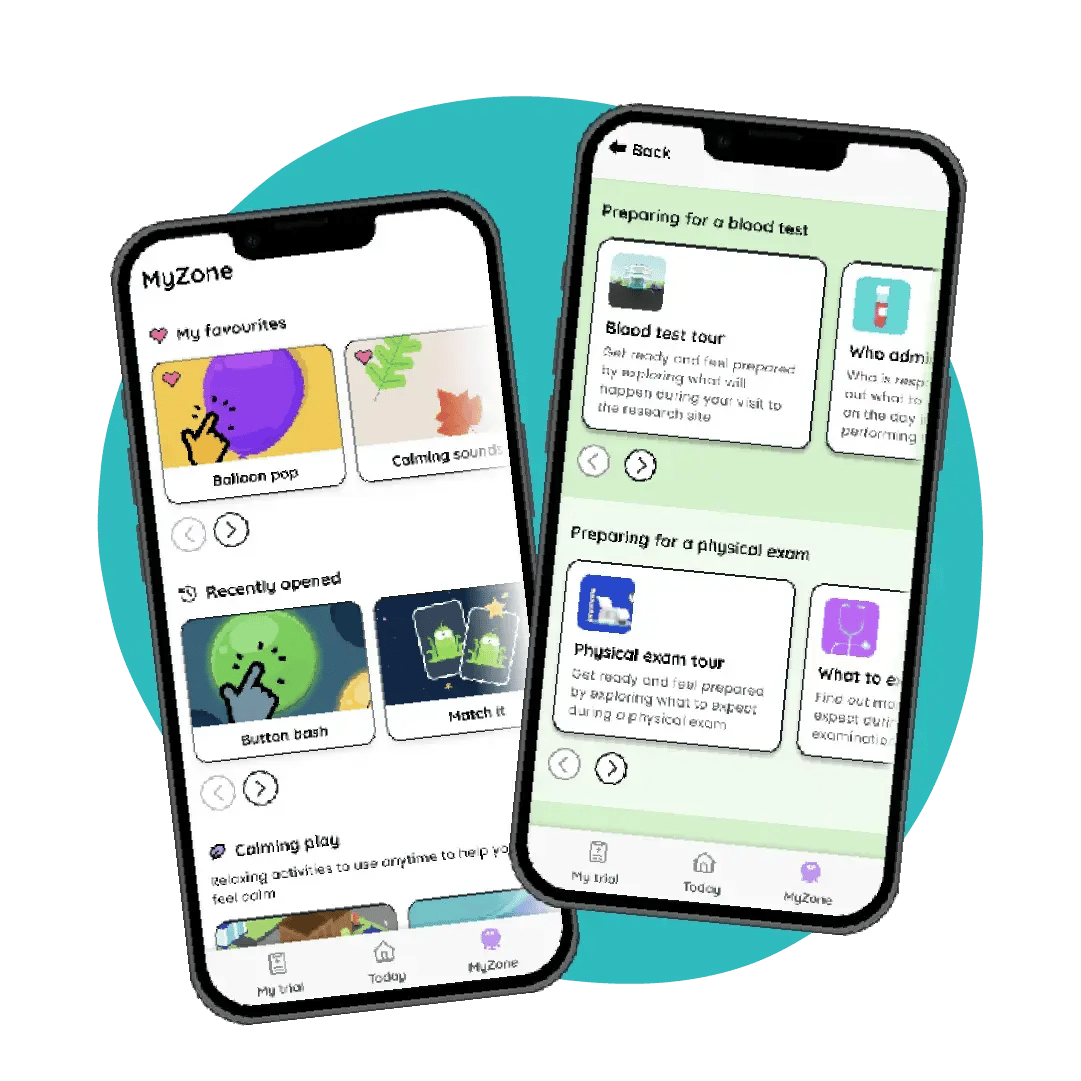
Retention
Support tools to develop coping skills and set expectations management throughout the trial to increase retention:
- Procedure education
- Virtual tours
- Preparation activities
- Soothing, calming, and distraction activities
Up to 45% reduction in dropout rates in trials using Little Journey
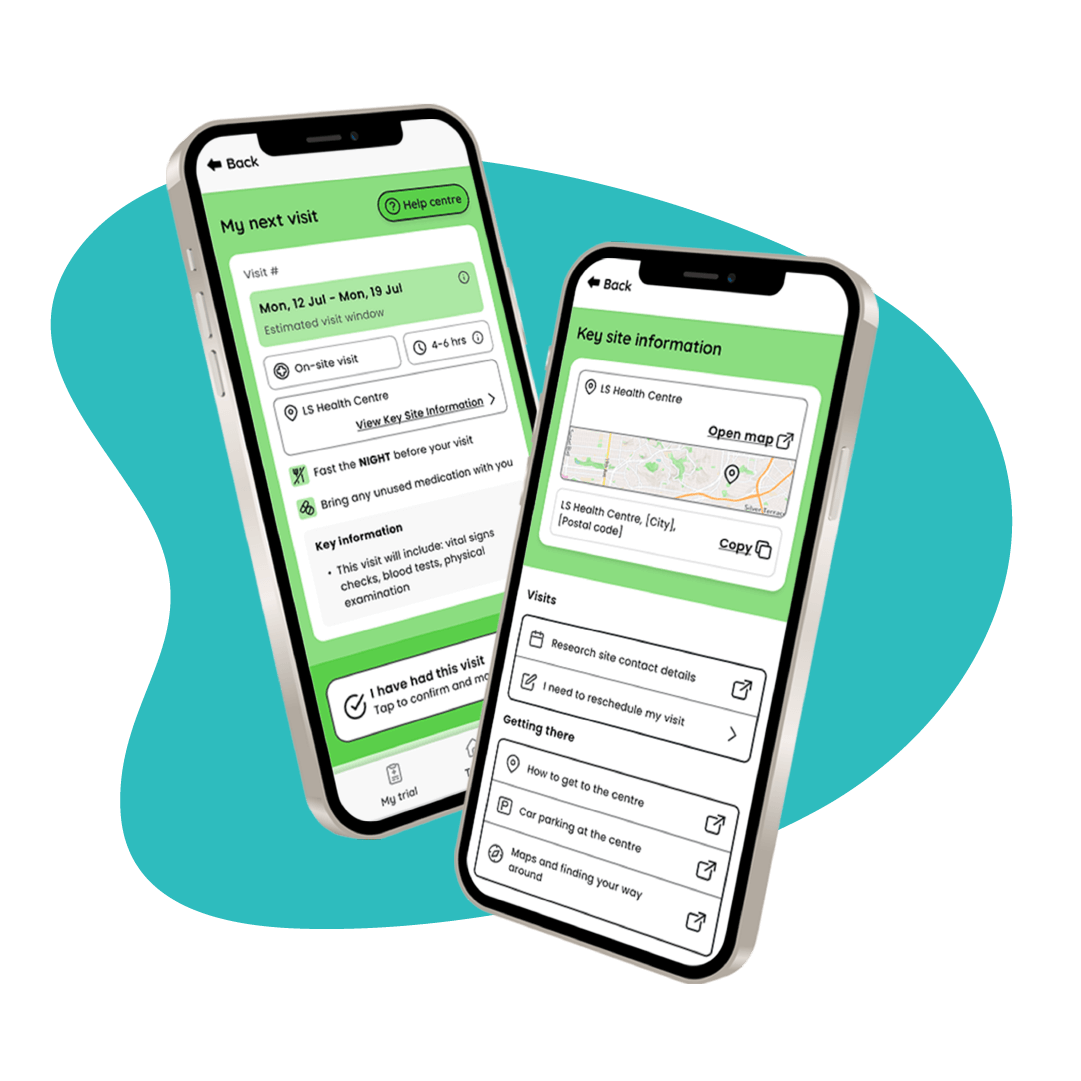
Adherence
Logistics tools to reduce trial burden and encourage protocol adherence:
- My Next Visit trial schedule timeline
- Reminders and prompts
- Key hospital information
- Checklists and notes
Hospital sites experience 42% fewer no shows and on-the-day cancellations when using Little Journey
Engagement
Education tools to improve clinical trial understanding and study awareness:
- Clinical trial FAQs
- Digitized PIS and trial documents
94% of families report increased understanding after using Little Journey
Delivery
Configuration and activation tools to streamline Little Journey's implementation into trial delivery:
- Automated ethics pack creation
- Site activation and training
- Platform analytics and reporting
95% uptake rate among sites offered Little Journey
Why pediatric trials fall behind
Pediatric studies face higher dropout rates, more delays, and greater discontinuation rates than adult trials. Existing retention strategies miss the real barriers families face:
-
Families don’t know what to expect
→ Low enrolment and early withdrawals lead to incomplete data. -
Study schedules clash with work, school and family life
→ Missed visits and protocol deviations delay timelines and drive up costs. -
Anxiety about invasive procedures and site visits
→ Higher refusal rates and cancellations, reducing adherence and data quality. -
Limited age-appropriate education for children
→ Resistance to participation, increasing dropout risk. -
Caregivers overwhelmed by logistics and lack of guidance
→ Missed tasks and poor adherence, creating gaps in data integrity.
Pediatric dropout rates are 20 - 50% across therapeutic areas
Every day of timeline delay costs upwards of $40,000
Discontinuation rates are 38% higher in pediatrics
Up to 40% of pediatric trials fail to achieve safety & efficacy end points
Your partner in pediatric trial innovation
We are pediatric specialists. Since being founded in 2017, we've focused exclusively in this space and built a deep expertise you can't get from one-off projects.
“Little Journey speaks to the gap I cannot speak to. Their power wheel is helping kids and the parents as well.”
“Little Journey are so diligent in co-creating with children and really bring their needs and voices into their solution.”

Rare Disease
Preparing and supporting children undergoing gene therapy
Little Journey partnered with a global pharmaceutical company to tailor our support solution for children with Duchenne Muscular Dystrophy (DMD) participating in gene therapy research.
Vaccines
Reaching 500,000 people with children's COVID-19 vaccine and testing education videos
NHS Digital, Public Health England, and NHS Test and Trace partnered with Little Journey to develop engaging content to explain COVID-19 vaccines and testing.



_screen%20use.png)

.png)