
Patient-centred clinical trials at scale
Trial Flow is a configurable platform designed to improve enrolment and retention across all therapy areas, regions and age groups. Grounded in behavioural science and real-world patient need, Trial Flow enables protocol aligned support to be delivered rapidly, helping to reduce drop outs, improve adherence and deliver studies on time and budget.

We are proud to support

50,000+ patients
all ages & caregivers
17+
countries worldwide
9
top global pharma & CROs
Improves enrolment
Drives engagement
Supports adherence
Reduces dropouts
How Trial Flow supports trial performance

Trial Flow analyses your protocol to configure and deploy optimal, study-branded patient support to enable rapid and consistent delivery across all therapeutic areas, patient populations, and languages, while supporting patient-centricity at scale.

Retention
Support tools to develop coping skills and set expectations management throughout the trial to increase retention:
- Procedure education
- Virtual tours
- Clinical trial FAQs
- Digitized PIS and trial documents
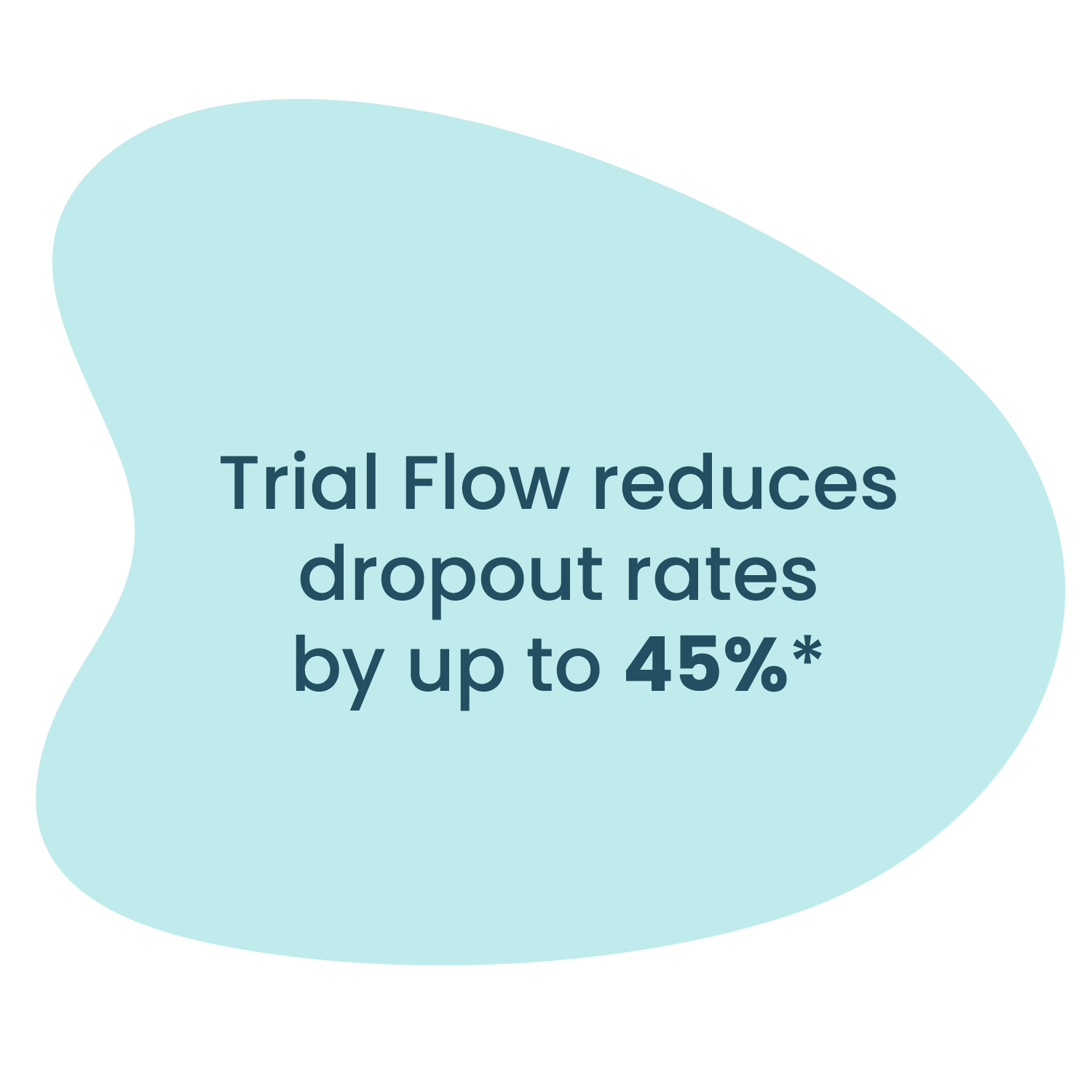
Up to 45% reduction in dropout rates in trials using Trial Flow

Adherence
Logistics tools to reduce trial burden and encourage protocol adherence:
- My Next Visit trial schedule timeline
- Reminders and prompts
- Key hospital information
- Checklists and notes
Hospital sites experience 42% fewer no shows and on-the-day cancellations when using Trial Flow

Engagement
Education tools to improve clinical trial understanding and study awareness:
- Preparation activities
- Soothing and calming activities
- Distraction activities
32% reduction in anxiety with Trial Flow

Data
Capture and analyse engagement to understand what's working and where:
- Centralised capture of all usage
- Monthly performance reporting
- Clear visibility into which tools and content drive the strongest outcomes
- Portfolio-level learning to inform future protocol planning
95% uptake rate among sites offered Little Journey
Why trials fall behind
Patients don't know what to expect
Study schedules clash with real life
Anxiety about invasive procedures
Limited age-appropriate content
Caregivers overwhelmed with logistics

All ages (children, adolescents, adults and caregivers)
Supporting patients and caregivers across all ages
Trial Flow delivers age-appropriate, protocol-aligned patient tools and content for children, adolescents, adults, and caregivers. Our Apps our configured to each trial and tailored to different needs, responsibilities, and motivations, ensuring a consistent, patient-centric experience across diverse populations.

Languages and localisation
Multi-language patient support at global scale
Trial Flow enables patient content include text, video and audio to be translated and localised across global trials, ensuring patients and caregivers receive consistent, protocol-aligned information in their preferred language. Our CMS enables rapid rollout across regions while maintaining governance and accuracy.



_screen%20use.png)

.png)