Clinical trials aren’t just protocols and data points, there are real people at the centre of each study. Consistent global challenges – like trial delays, delivery costs, and data quality – which slow the advancement of paediatric medicine grow out of the day-to-day challenges faced by research staff and the patients they serve.
As a former paediatric research nurse, I’ve experienced these pressures firsthand. From the families navigating complex trial demands to the site teams doing their best to deliver exceptional care while managing a heavy operational load. Today, in my role as Site Success Manager at Little Journey, I bridge those worlds, ensuring our technology truly addresses these root challenges for site staff and participating families.
Understanding the human factors
During my time managing paediatric neuromuscular trials, I worked closely with children and families involved in complex rare disease studies.
Many of these families arrived at our site carrying more than just logistics, they carried hope, uncertainty, and the weight of difficult decisions. I also remember the hard work of my colleagues behind the scenes to coordinate multiple trials, navigate multiple systems and manage multiple stakeholders, quite often with minimal support.
These experiences shaped how I view trial delivery today:
Successful studies depend on more than protocol adherence. They need compassion, communication, and tools that truly support families, participants, and the people delivering the trials.
Why pharma needs tech that speaks to sites
Investment in trial innovation is growing. This is great news. But too often sponsor and CRO-led initiatives overlook the frontline reality of trial management:
- Fragmented systems: Study coordinators can be running 5+ trials on 15+ platforms, each with its own user interface and training requirements.
- Cognitive overload: Research staff switch between trial visits, coordinating the multidisciplinary team, entering data and resolving data queries, phone calls, and queries from families, with little margin for error.
- Emotional Labor: Calming a child’s fear over a blood test or walking a parent through complex trial discussions is as critical as data entry—and just as draining.
If tech adds another login, another manual upload, or another unclear alert, it becomes a blocker, not a booster for trial efficiency.
Delivering boosters not blockers
I joined Little Journey because I saw an opportunity to make a broader impact on paediatric clinical research, reducing trial burden for site teams and families. I know how valuable it is when digital tools genuinely lighten the load, not add to it.
For me, that’s what makes Little Journey’s platform so powerful - it’s built to meet the needs of both patients and providers:
- Personalised support tools: Content in our family-facing app is tailored to the child’s age, the trial protocol, and the research site. We can include 360 images of rooms families will visit and key site information.
- No portal or setup for site teams: My role focuses on implementing Little Journey with trial sites globally. We take a “white glove” approach, offering remote or in-person site activation sessions to remove the platform setup burden from sites.
- A dedicated site success manager: I continue to work with the site after implementation for the duration of the trial, answering any questions and supporting patient onboarding.
I’m incredibly proud of the site trust, loyalty, and advocacy we’ve built at Little Journey. Our NPS score with our site partners is 83. That’s considered world-class and means the sites currently active with Little Journey are highly likely to recommend us to a colleague.
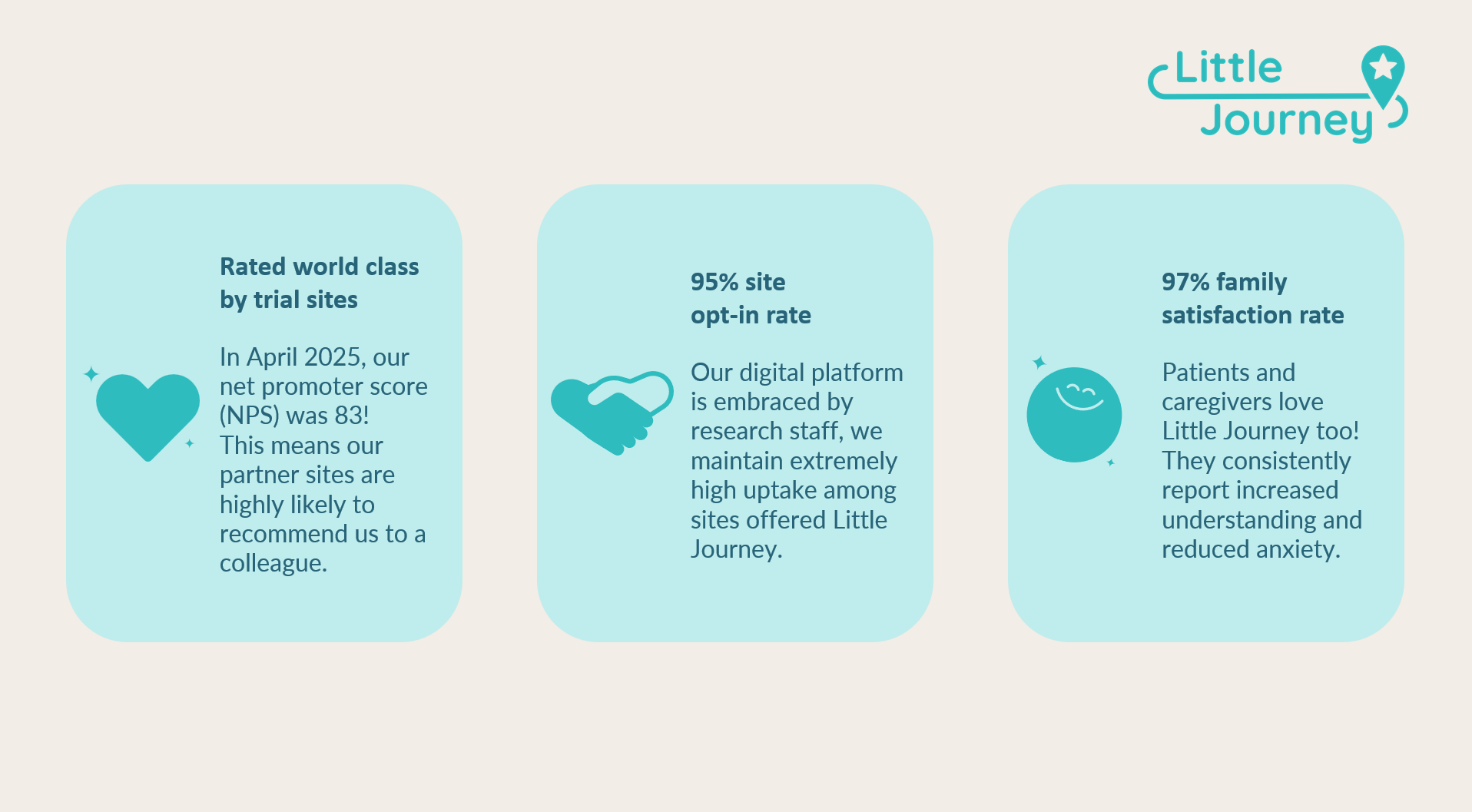
One U.S. research coordinator shared:
“I think the app is wonderful. The way it uses age-appropriate language for the patients is great. It is user-friendly, and I strongly believe it alleviates anxiety for patients attending our site, as everything is explained beforehand.”
Turning empathy into impact
When families feel prepared, they’re less anxious and more engaged. When sites feel supported, they can focus on care, not admin. And when sponsors and CROs invest in meaningful digital engagement, they see improved retention, fewer protocol deviations, and better data quality.
I bring that same empathy I felt as a research nurse into every site I support, ensuring both trial delivery and participant experience are set up for success.
Let’s keep improving the trial experience
If you're a site professional, sponsor, or CRO looking to deliver trials that are not only efficient but truly participant- and site-centred, we’d love to connect.
Speak to Asyah, on LinkedIn or at asyah.chhibda@littlejourney.health, to learn more about site success at Little Journey.
Or, book a demo to see how our platform lightens the load for research sites.